PoSM Lab Github

Physics of Soft Matter Lab at Harvey Mudd College
Organisms with Ultra-Fast Biomechanics
Organisms that store and release elastic energy can achieve remarkably fast movements. This page contains examples of organisms that do this, and more information about each of them.
To contribute to this page, choose an organism from the list below and add information to organisms.md in the posmlab Github repository. Some starting references are provided as Google Drive links in each section. If you find other research papers, be sure to save them to the posmlab Google Drive “Papers” folder and link to them here. The organisms listed are mostly from Table 1 of Ilton et al Science 2018, but the supplementary info of that paper has many more examples with references. If you’re looking to add a new organism, look at the table starting on page 30 of the supplemental information and add it here!
List of Ultra-Fast Organisms
(Organism - Movement)
Fungi - Ballistospore ejection
Bunchberry dogwood - Pollen ejection
Trap-jaw ant - Mandible strike
Aquatic bladderworts - Suction trap
Venus fly trap - Snap buckling
Mantis shrimp - Appendage strike
Snow Flea - Catapult Jump Mechanism
Cone Snail - Ballistic Tooth Propulsion
Hydra
Nematocyst discharge
Stinging cells (nematocytes) in jellyfish, sea anemone, and hydra (all from the phylum Cnidaria) discharge their contents at extremely fast rates. For example, this video of many nematocysts discharging simultaneously or this video of a single nematocyst discharge show what the process looks like.
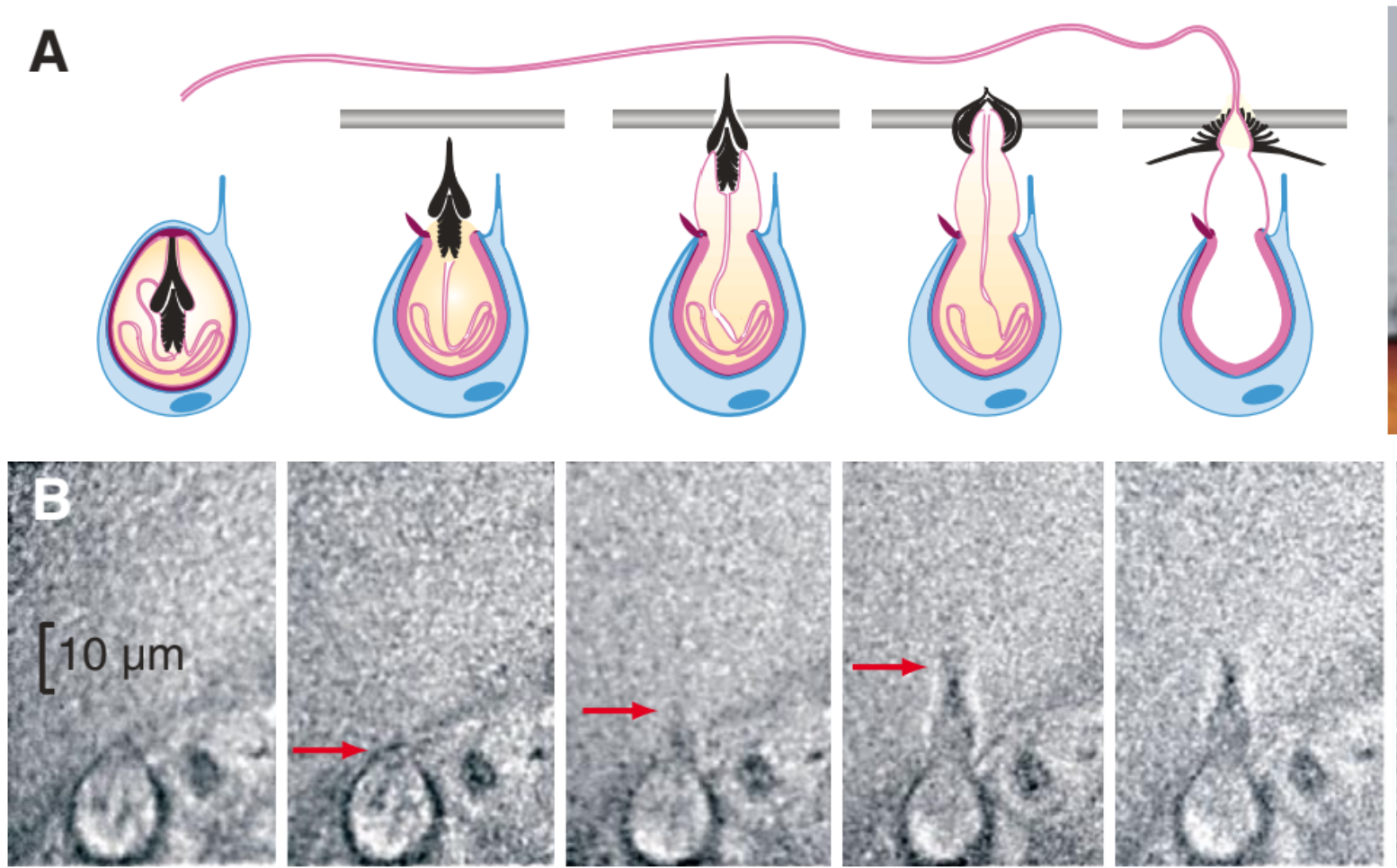
High speed imaging of Hydra from Nüchter et al. Curr Biol 2006 show this discarge can occur in 700 nanoseconds, and the stylet (barbs) accelerate at 5 million g’s of acceleration:
Part of Figure 1 from Nuchter et al. (A) Schematic of a nematocyst discharge. The stylet (black) is rapidly accelerated towards the prey (silver horizontal line). (B) Sequence of high speed images showing this process experimentally. The nematocst is roughly 10 um in diameter, and the time between each image is 500 ns.
Summary of Kinematic Peformance from Nuchter et al
| Kinematic Metric | Hydra performance |
|---|---|
| Duration | 700 ns |
| Max. Velocity | 37 m/s |
| Max. Acceleration | 5.3 X \(10^7\) m/s\(^2\) |
Wikipedia has a good description of nematocytes in the section on Cnidocytes. The possible mechanisms for the rapid release of energy in this system are outlined in that section as well:
- Rapid contraction of elastic collagen-like fibers in the walls of the capsule
- The thread acts like a coiled spring that extends rapidly when released
- Chemical changes in the capsule contents may cause them to expand rapidly by polymerization.
- Chemical changes in the liquid in the capsule make it a much more concentrated solution, so that osmotic pressure forces water in very rapidly to dilute it.
Before discharge, osmotic swelling causes a 30% increase in the volume of the capsule. Through exocytosis (a form of active transport which allows the transportation of molecules outside the cell), the kinetic energy – from elastic stretching, due to the significant volume increase – stored in the capsule wall is released with an acceleration of about 5.4 X \(10^7\) m/s\(^2\) (also seen in the kinematics v. performance table above). After discharge, the volume of the capsule is substantially decreased. The capsule walls consist of a matrix of minicollagens, and while these minicollagens can account for the strength required for the capsule walls to withstand the pressure of osmotic swelling priot to discharge, the elastomeric proteins resposible for storing the energy required for the impressive kinetics of discharge have yet to be identified. (For further reading, see Beckmann et al. BMC Biology 2015.)
Hydras typically prey on crustaceans – it is the hard external shells of these prey animals that necessitate the incredible kinematic profile of the nematocyst, since the extremely small mass of the ejected stylet (~1 ng) would be incapable of penetrating them with more mundane accelerations. And even with top accelerations in the area of 50 million m/s, the kinetic energy of the stylet evens out to only around 0.1 micro-Joules – it is the physical dimensions of the stylet that provide the last piece of the puzzle, with their miniscule tips allowing for a much higher pressure than one might expect from their modest kinetic energies. In fact, with estimates placing the maximum pressure at upwards of 7 GPa, the pressure profile of a stinging nematocyst is comparable to that of a bullet. (Data drawn from Nuchter et al.)
For further rearding, Koch et al. J Cell Sci 1998 has more details about the structure of the components of a nemacoyst.
Fungi
Ballistospore ejection
There are 30,000 species of mushroom, yeasts and pathogenic rusts/smuts make use of a surface tension catapult to spread its spores to reproduce (hence the name ballistospore combination of ballistic and spore). Due to fungi’s lack of musculature, the evolution of the dispersal devices varies greatly, but all rely on some form of energy build up to compensate. These spore dispersions have over 10,000g of initial acceleration, which is larger than any other known animal or plant.
The Paper (Pringle et al. Mycologia 2005) shows that the ejection of the spore occurs when the Buller’s drop (a droplet of water that forms at the base of the spore near the sterigma) combines with a film of water on the spore itself, and releases the energy stored in its surface tension (this process is called fusion where the water coalesces just before ejection). The drop and the water collected on the surface contain certain sugars and polyhydric alcohols to cause partial hydrophobicity. This prevents the collapse until the drop has reached a critical size to allow for proper dispersion. Figure 1 shows the images taken at high speed of the process taking place. Figure 2 depicts the mechanism that drives the process.
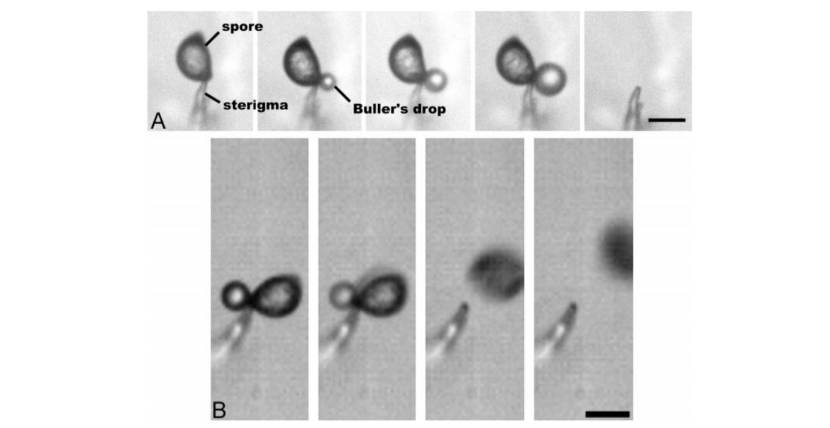 Figure 1:
A. Spore sits on top of the sterigma (a part of the fungus). Displays the growth of the Buller’s drop and final frame is after the spore and the drop have disappeared (10 s between each frame).
B. Images of Buller’s Drop and ballistospore using a high-speed camera (10 μs between each frame).
Figure 1:
A. Spore sits on top of the sterigma (a part of the fungus). Displays the growth of the Buller’s drop and final frame is after the spore and the drop have disappeared (10 s between each frame).
B. Images of Buller’s Drop and ballistospore using a high-speed camera (10 μs between each frame).
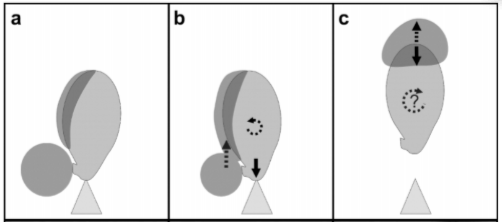
Figure 2: Illustrates the surface tension catapult.
A. Buller’s drop forms at the base of the spore, energy is stored here in the form of surface tension. B. Buller’s drop touches the side of the spore and merges with the fluid, releasing the tension, causing an upward movement, and a downward force on the sterigma and a bit of rotation. This mechanism mimics how we jump as a downward force is applied before release to cause the system to propell itself upwards. C. Once the Buller’s drop reaches the tip of the spore it stops abruptly due to surface tension preventing it from leaving the spore. This causes the spore to be shot upward along with the droplet.
There are two different sources of momentum for the spore from the Buller’s drop. The first is the initial coalescence causing a downward force and rotation, releasing the initial built up surface tension. And the second, which is the much more significant aspect, is that the drop then travels up the spore gaining momentum until the very top of the spore. When it reaches the top, the surface tension stops the drop and it pulls the spore forward with the drop’s momentum causing a catapult effect.
To see the action check out the following videos:
Summary of Kinematic Performance from Pringle et al. Mycologia 2005
| Kinematic Metric | Ballistospores’ performance |
|---|---|
| Duration | 10 μs |
| Max. Velocity | 1.57 m/s |
| Max. Acceleration | \(1.2\) X \(10^5\) m/s\(^2\) |
How it compares to other organisms:
It out does the mantis shrimp in terms of acceleration but is dwarfed by the hydra and in terms of initial velocity the bunchberry dogwood pollen catapult does much better.
Source: Pringle et al. Mycologia 2005
Bunchberry dogwood
Pollen ejection
Bunchberry dogwood (Cornus canadensis) is a plant that grows in dense carpets in the spruce-fir forests of the North American taiga [1]. Bunchberry grows clusters of up to ten berries, from which the plant gets its name [2]. Bunchberry flowers grow in groups (inflorescences) surrounded by four white bracts, as seen in Figure 1a. In Figure 1b, we can see that an individual flower has four petals (which droop down in the second image) and four stamens, each composed of a long filament and an anther on top which stores the pollen.
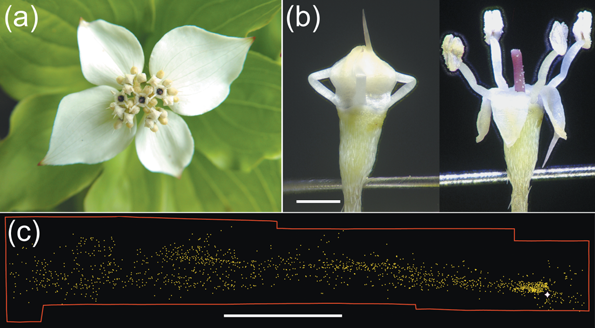
Figure 1: (a) An inflorescence of 30 bunchberry flowers. (b) Close-up of a single mature flower before and after opening. Elastic energy is stored in the bent filaments, which protrude from between the petals in a closed bud. Scale bar = 1 mm. (c) Pollen distribution from one flower triggered in a closed room showing that even minor air currents can carry pollen. White star shows location of flower; each yellow dot represents a pollen grain or clump of grains. Scale bar = 5 cm. [3]
In a young flower bud, the filaments are short and the petals are fused along the edges, completely enclosing the stamens [3]. As a flower matures, the stamens lengthen faster than the petals and become bent, as seen in the first image in Figure 1b. Elastic energy is stored in the bent stamens in the form of turgor pressure—pressure that arises from the plant cells being full of water, which tends to straighten out the stamens [3].
One of the four petals typically has a larger part that acts as a trigger, which can be seen in Figure 1b [3]. The flower will also eventually burst open on its own. When the flower opens, the plant tissue holding the petals together is torn and the elastic energy stored in the stamens is released, catapulting the pollen up into the air. The force required to trigger the opening (0.1–0.5 mN) favors large pollinators, such as bumblebees, that move quickly between inflorescences and excludes smaller and slower visitors such as ants [1]. Flowers that open on their own can also spread pollen via the wind, as shown in Figure 1c [1].
Here is a video of the pollen being released: https://youtu.be/kDbHMDIYKzw.
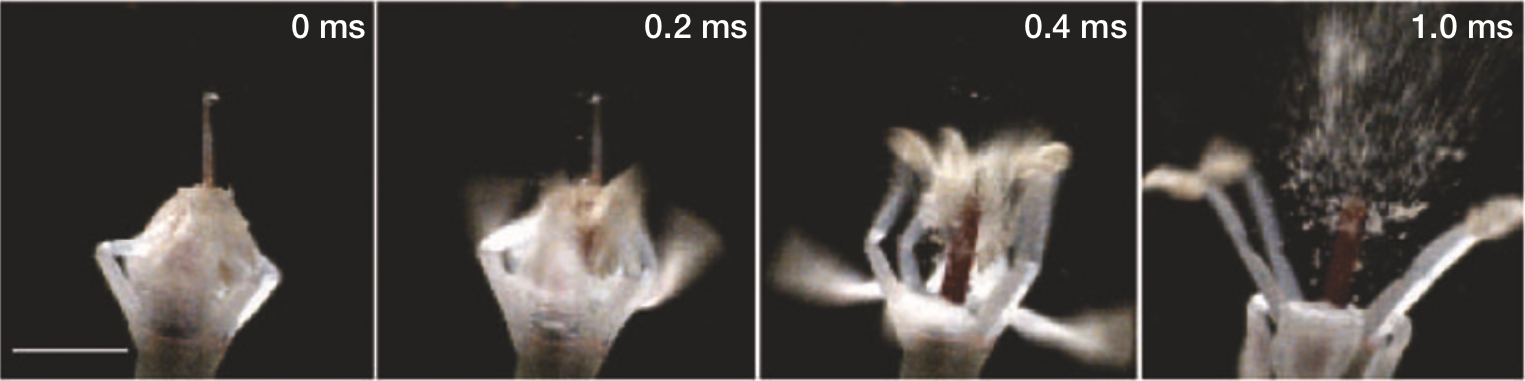
Figure 2: Bunchberry flower opening, recorded on video at 10,000 frames per second. Time elapsed is indicated. First frame shows a closed flower with four petals fused at the tip, restraining the stamens. Blur represents the distance moved in 0.1 ms. Scale bar, 1mm. [1]
In this process, the stamens reach velocities of 3.1 ± 0.5 m/s and accelerations of 24,000 ± 6,000 m/s\(^2\) (2,400 g), while the petals reach velocities of 6.7 ± 0.5 m/s and accelerations of 22,000 ± 6,000 m/s\(^2\) (2,200 g) [1]. The pollen granules reach a height of 2.5 cm (more than 10 times the height of the flower) and can then be dispersed by the wind.
In bunchberry flowers, the stamen functions similarly to a miniature medieval trebuchet, a special catapult that maximizes throwing distance by having the payload (in this case, the pollen in the anther) attached to the throwing arm (the filament) by a hinge or a flexible strap [1]. In fact, Whitaker et al. have shown that in bunchberry flowers, anther rotation about the filament tips delays the release of pollen until the vertical speed is maximized [3].
Summary of Kinematic Performance
| Kinematic Metric | Bunchberry Flower Performance [1] |
|---|---|
| Duration | < 0.5 ms |
| Max. Velocity | 3.1 ± 0.5 m/s |
| Max. Acceleration | 24,000 ± 6,000 m/s\(^2\) |
Sources
[1] Edwards et al Nature 2005
[2] https://www.thespruce.com/bunchberry-shade-ground-cover-2132948
[3] Whitaker et al Funct Ecol 2007
Trap-jaw ant
Mandible strike
The mandible strike of trap-jaw ants serve as methods to avoid or eject predators and capture prey. During a mandible strike, trap-jaw ants close their mandibles at incredible speeds over short durations in specific orientations and strike surfaces to fit their desired function (avoiding predators,… ). Their common propulsion behaviors are named “bouncer defense” and “escape jump.” Bouncer defense is when trap-jaw ants strike at predators while propelling themselves away. Escape jumps, on the other hand, is when trap-jaw ants strike at a substrate and vertically propel themselves to escape predation.
Image of Trap-Jaw Ant from Patek et al PNAS 2006
Figure 3 from Patek et al. (a) Shows a trap-jaw ant using bouncer defense against an intruder (plastic strip). (b) Shows a trap-jaw ant using escape jump.

Summary of Kinematic Peformance from Patek et al
| Kinematic Metric | Trap-Jaw Ant performance |
|---|---|
| Duration | 0.13 ms |
| Max. Velocity | 64.3 m/s |
| Max. Acceleration | \(10^5\) X \(g\) m/s\(^2\) |
For further reading, Patek et al PNAS 2006 has more details about the mechanisms of a trap-jaw ant’s mandible strike.
Plant louse
Jump
Louse is singular for lice! This summary is about jumping plant lice (psyllidae), which are a family of small plant-feeding insects that tend to be very host-specific.
Here is a video of one such species walking around. Unfortunately, catching its jump with a normal camera is probably close to impossible, given how fast they jump. However, in this paper, researchers have used high-speed cameras to capture the following images of a specific species of plant lice”
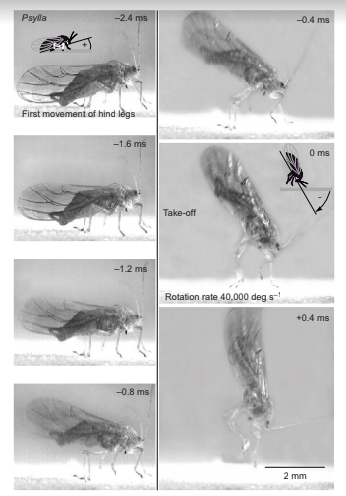
Images of a jump by Psylla alni, viewed from the side and capturedat 5000 Hz, each with an exposure time of 0.05 ms. The images are arranged in two columns, with the bottom left-hand corner of each image providing a constant reference point in this and in Figs 5, 7–9. The hind legs started to move at –2.4 ms, and the continuing depression of the hind trochantera raised the rear of the body so that the middle legs lost contact with the ground and the head pitched forwards. Once airborne, the body rotated rapidly in the pitch plane. The cartoons show how the angle of the body relative to the ground was measured when the head was pointing upwards (frame –2.4 ms) and then downwards (frame 0 ms).
Summary of Kinematic Peformance from Burrows
| Kinematic Metric | Lice performance |
|---|---|
| Duration | 0.9 - 1.7 ms |
| Max. Velocity | 1.1 - 2.7 m/s |
| Max. Acceleration | ~2 X \(10^3\) m/s\(^2\) |
The mechanism for the rapid release of energy is known in detail. Generally, these lice jump similarly to other insects, with the exception that they have a distinct take-off position in which the head points downwards and the front supports the bug, similar to a person doing a hand-stand.
For further rearding, Burrows JEB 2012 has more details about the physics of plant lice jumps and the relevant anatomy.
Aquatic bladderworts
Suction trap
Aquatic bladderworts are carnivorous plants plants that use suction traps to catch small prey. Suction is created by a very fast opening and closing of a trapdoor, which releases stored elastic energy. This trapping mechanism is one of the fastest known plant movements.
The suction trapping mechanism has two stages: a slow phase, and an ultra-fast phase. In the slow phase, the internal glands slowly pump water out of the trap. This reudces the hydrostatic pressure, which increases the elastic energy stored in the walls of the trap. Once the prey stimulates the trigger hairs on the watertight trap door, the ultra-fast phase is initiated. The door opens, causing a rush of water to flow into the trap, pulling in the prey. Then the prey is slowly broken down by a cocktail of digestive enzymes.
Images of the slow and ultra-fast phases of the suction trap:
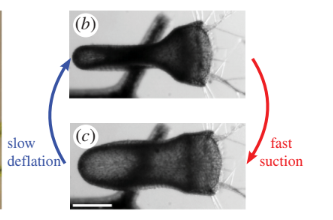
High speed cameras were used to image this process. The fast opening and closing of the door, with an abrupt change in shape, strongly suggests that the under-lying principle is a buckling of an elastic valve, which is triggered by the trigger hairs on the trap door.
Schematic of trap door deformation:
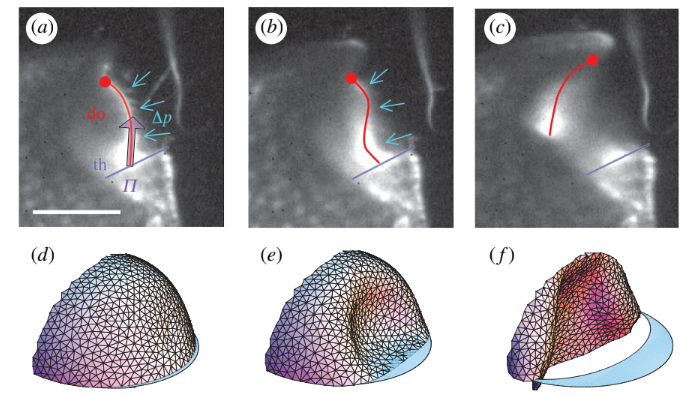
Simulations of the mechanism show that the the stiffness of the walls of the trap must be optimized to be soft enough to deform sufficiently, but also stiff enough to overcome viscous disspation and move fast enough to catch the prey.
The suction mechanism of these carnivorous plants is fascinating, and also useful. Since these traps are autonomously repetitive, this research the potential to inform the design of microfluidic devices that must act repeatedly.
| Kinematic Metric | Aquatic Bladderwort performance |
|---|---|
| Duration | < 0.5 ms |
| Max. Fluid Velocity | 1.5 m/s |
| Max. Acceleration | 600g |
Vincent et al RSPB 2011 has more details about the suction mechanism of aquatic bladderworts.
Froghopper
Jump
Froghoppers are a diverse and abundant family of insects known for their incredible jumping capabilities; jumping as high as 115 times its body length. The jumping mechanism for froghoppers are similar to those of locusts and fleas that rely on elongated hind legs to provide propulsion upwards. However, whereas the hind legs of locusts are on the scale of its body length; the hind legs of froghoppers scale to only half of its body length and whose mass contributes only about 2% to its total mass. Therefore, froghoppers must have a unique mechanism that enables them to achieve high jumping heights. This is achieved through a combination of precise body orientation and careful movement of the muscles in the hind legs that provide a large acceleration up to 550g. The froghopper positions himself by raising its hind legs and rapidly depressing its trochantera which sends the insect airborne.
| Kinematic Metric | Froghoppers’ performance |
|---|---|
| Duration | 0.875 ms |
| Max. Velocity | 4.7 m/s |
| Max. Acceleration | 5500 m/s\(^2\) |
Venus fly trap
Snap buckling
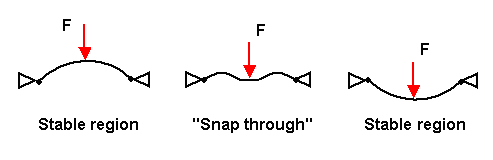
The rapid closure of venus fly traps is achieved using a method called snap buckling, where a system “snaps” from one stable state to another stable state. Here’s a diagram that visualizes snap buckling.
For reference, here is a labeled diagram of the parts of a venus fly trap.
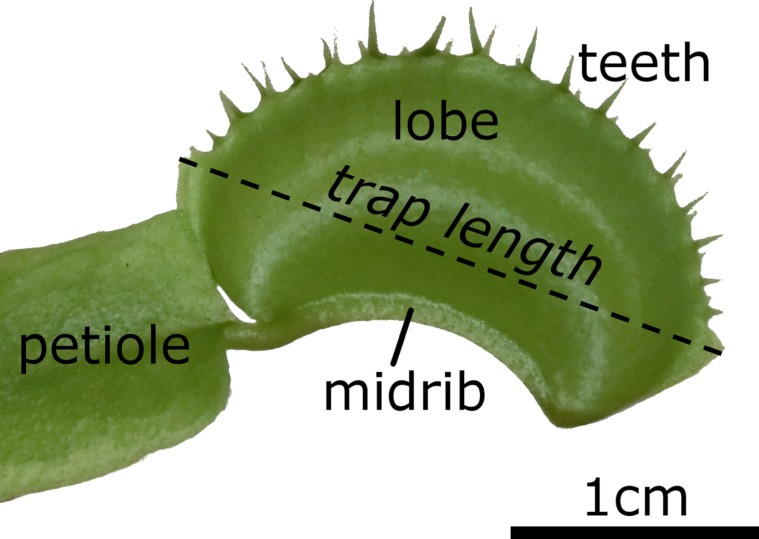
In the open state, the lobes of the venus fly trap are convex (when viewed from the inside), while in the closed state, the lobes are concave.

When the venus fly trap goes from the open state to the closed state, the lobes undergo snap buckling from convex to concave. Here’s a video showing it close up.
The snap closure of venus fly traps usually occur in less than half of a second, though the snap time varies from about 0.2 to 0.8 seconds. Since venus fly traps are also found to grow underwater, researchers timed the closing of submerged venus fly traps, and found that the closing time underwater was not significantly different from the closing time on land.

Additionally, tests involving ink droplets in the water showed that there was no significant outflow of water upon closure, meaning that venus fly traps could effectively catch underwater organisms without failing due to water being forced out, though this has yet to be studied in depth.
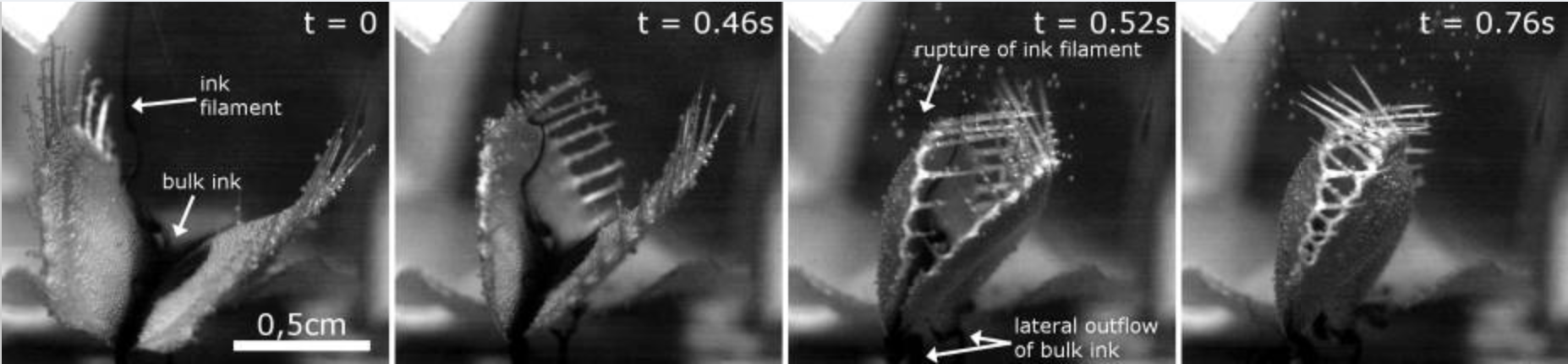
For further reading, Poppinga et al Beilstein J Nanotechnol 2016 has more information about the snap buckling closing mechanism of venus fly traps.
Mantis shrimp
Appendage strike
Mantis Shrimp, while technically neither a mantis nor a shrimp, are actually another type of crustaceans more technically known as stomatopods. Mantis Shrimp, though only a few inches in length, use Latch Mediated Spring Actuation to fire off deadly punches to protect their territory and hunt prey. Their punches are so strong, they have even been know to break the masks of divers who get too close to their dens. However, these strikes are more commonly used to break through the shells and exoskeletons of their prey. The force of their strike is enough to produce what is known as a cavitation bubble. Inside these bubbles, the pressure is so low that the water vaporizes. When these bubbles collapse, they emit energy underwater in the form of heat, light, and sound.
Types of Mantis Shrimp
When looking at the anatomy of Mantis Shrimp, we see that the appendages they use to punch can be categorized into two different types: spearers and smashers. The differentiation is in the style of dactyl, as shown by McHenry et al:
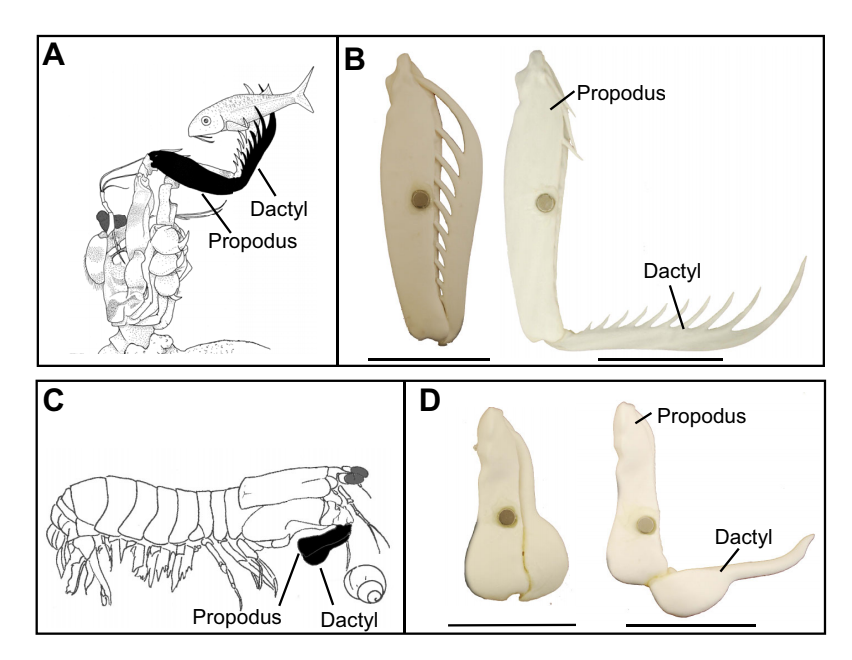
Kinematics
For a single spear-type female of the species Coronis scolopendra the following data was collected by McHenry et al:
| Kinematic Metric | Coronis Scolopendra Performance |
|---|---|
| Time to Max. Linear Speed | .89 - 2.37 ms |
| Max Linear Speed | 2.4 - 3.3 m/s |
| Max. Linear Acceleration | 2500 - 5300 m/\(s^2\) |
For further information, see the full paper by McHenry et al.
Frog
Jump
The Australian Rocket Frog achieves the second longest relative jumping distance for any anuran, 55.2 body lengths for one individual. They also achieve the highest published anuran values for isolated net mean power output, hindlimb length to snout-vent length ratio, and relative hindlimb muscle mass. For the individual that jumped the farthest, it was found that the mean power output expended during takeoff was about 3 times larger than the estimate of available muscle mass output.
Figure 2 from Anderson. Changes in (A) Acceleration, (B) Velocity, (C) instantaneous body-mass-specific power output during a typical jump at 25°C for an Australian rocket frog.
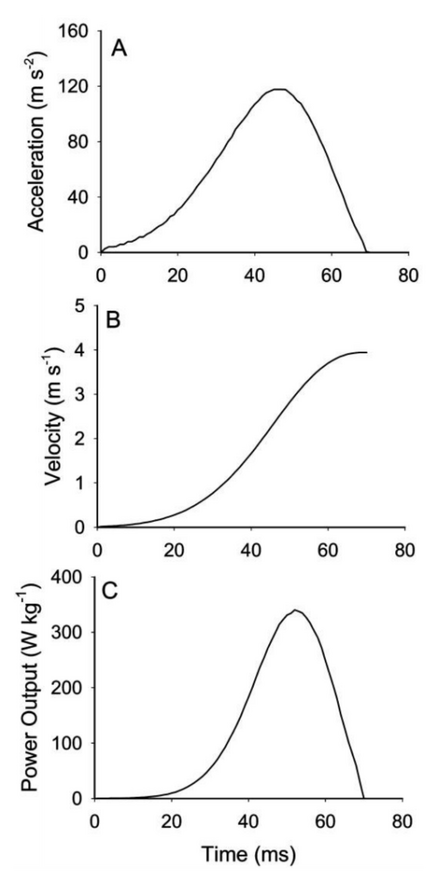
Kinematics
The maximum jumping performance was determined for eight adult male Australian striped rocket frogs was collected by James & Wilson Physiol Biochem Zool 2008:
| Kinematic Metric | Australian striped rocket frog Performance |
|---|---|
| Contact time (ms) | 54-81 |
| Max. force (N) | 0.238-0.824 |
| Max. jump distance (m) | 0.71-2.17 |
| Max. velocity (m/s) | 2.59-4.52 |
| Max. acceleration (m/\(s^2\)) | 86-140 |
| Max. instantaneous power output (W/kg body mass) | 169-455 |
| Average power output during takeoff (W/kg jumping muscle mass) | 318-747 |
For further informaion, see James & Wilson Physiol Biochem Zool 2008
Chameleon
Ballistic Tongue Projection
Chameleons project their tongues up to 2 times their body length in order to capture prey. Especially in smaller species, their tongue projection has evolved in order to minimize energy loss and maximize the efficiency of the feeding apparatus. Variation in tongue length, body length, and most notably snout-vent length (distance between cloaca and where the jaw joins, the jaw symphysis), result in the variations in tongue mechanism function. The tongue quick projection and retraction is acheived by 2 muscles, the accerlator muscle and the hyoglossus that are activated sequentially, as the tongue folds in an accordion-like manner.
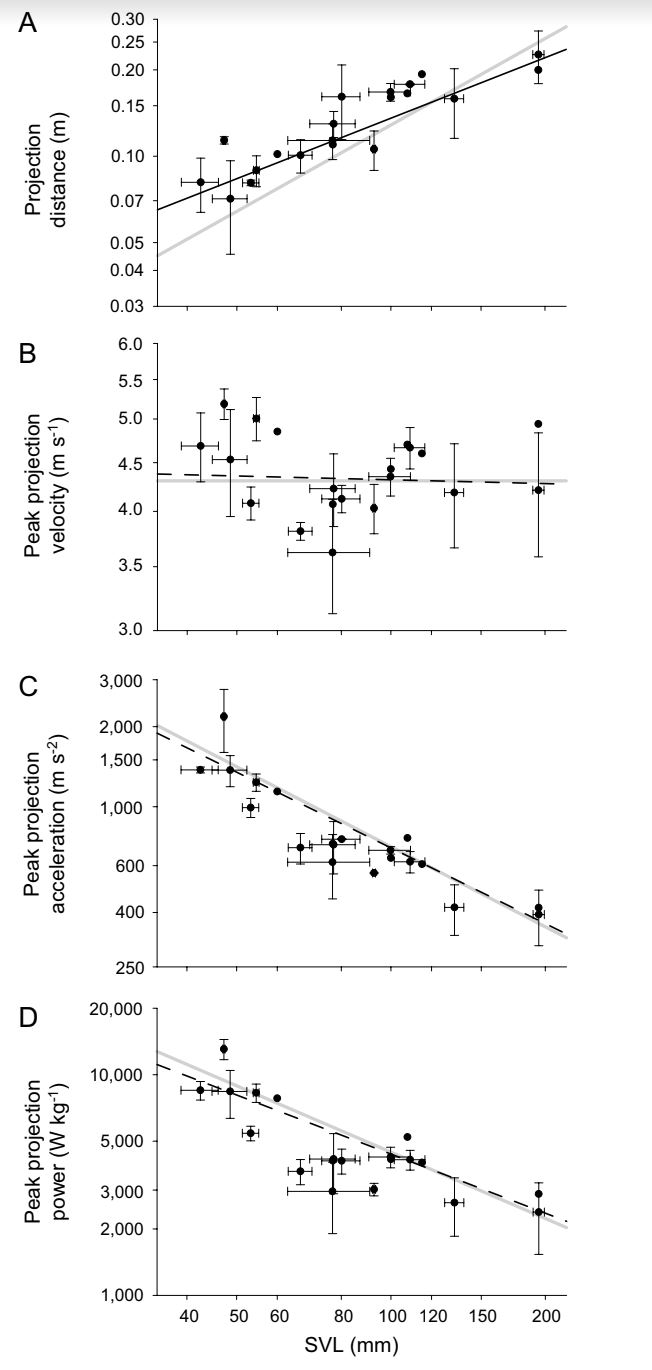
Figure 2 from Anderson. (A) Peak projection distance, (B) Peak projection velocity, (C) Peak projection acceleration, (D) peak mass/specific power output with respect to snout-vent length.
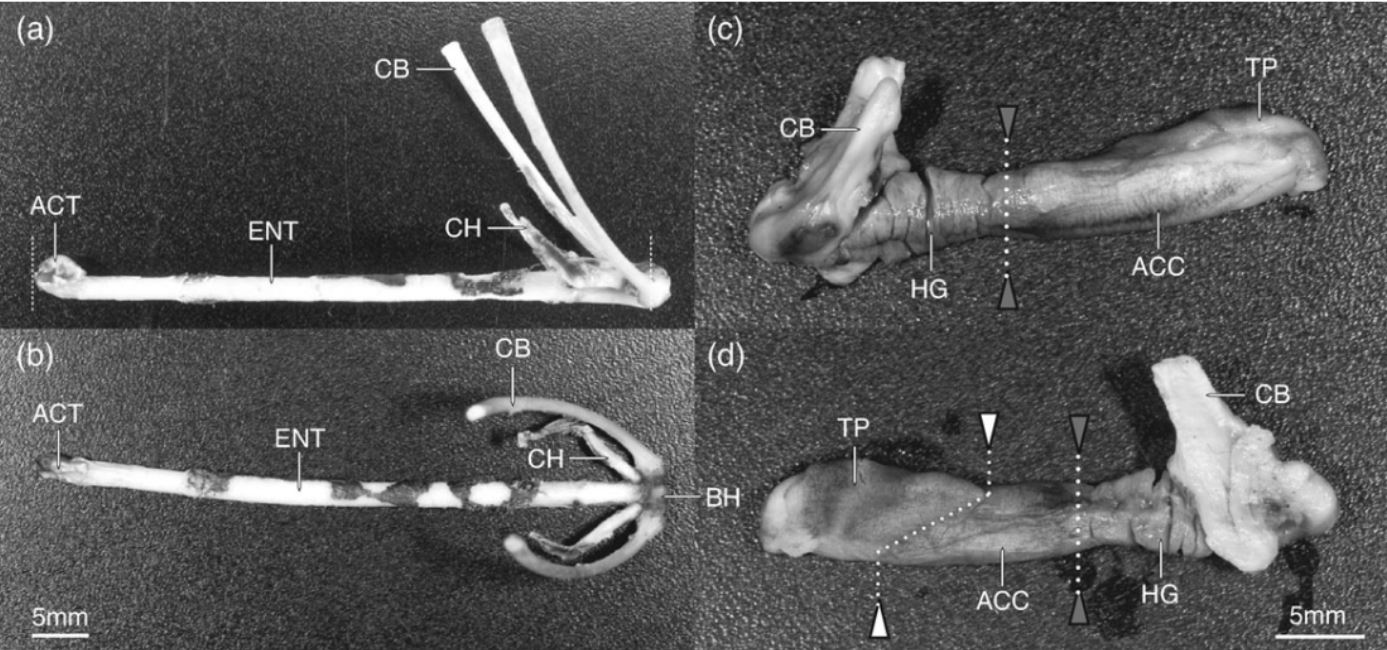
Figure 1 from Anderson et Al. demonstrating the skeletal and muscular components of the projection and retraction mechanism
Summary of Kinematic Peformance from Anderson
The kinematic performance of the chameleon tongue mechanism varies between species, with smaller species demonstrating the shortest durations, and highest accelerations and power outputs. The max. velocity acheived is proportional between species. Data was scaled by SVL, or snout-vent length of the species.
| Kinematic Metric | Chameleon Tongue Performance |
|---|---|
| Duration | 9.7 - 54.6 ms |
| Max. Velocity | 2.91 - 5.41 m/s |
| Max. Acceleration | 286 - 2,590 m/s\(^2\) |
| Max. Power Output | 1410 - 14040 W/kg |
For further reading, Anderson et al J Morphol 2012 describes the differences in tongue projection between the species of chameleon.
Locust
Jump
Locusts jumps, while not having the most impressive performance stats for acceleration or power output, are a well-studied spring-latch system that have been used as a model for many miniature jumping robots.
| Kinematic Metric | Locust performance |
|---|---|
| Duration | 20 - 30 ms |
| Max. Velocity | 3.2 m/s |
| Max. Acceleration | 180 m/s\(^2\) |
| Max. Power Output | 450 W/kg |
For further reading, Bennet-Clark JEB 1975 and Chen et al Acta Mechanica Sinica 2014 for further analysis with high-speed video.
Caddis Flies
Jump
Two distinct jumping strategies were found in caddis flies. The first strategy was performed by depressing and extending the middle and hind pairs of legs at the same time, while the second strategy involved wing movements in addition to the same leg movements before take-off. Due to the kinematics it was determined that both of these movements were only produced by direct muscle contractions and as such did not use any power amplification or energy storage.
Figure 3 from Anderson. Jump by L. marmoratus in which only the legs participate. Jumps in which the wing movements are included would result in a higher angle between the body and the surface at take-off.
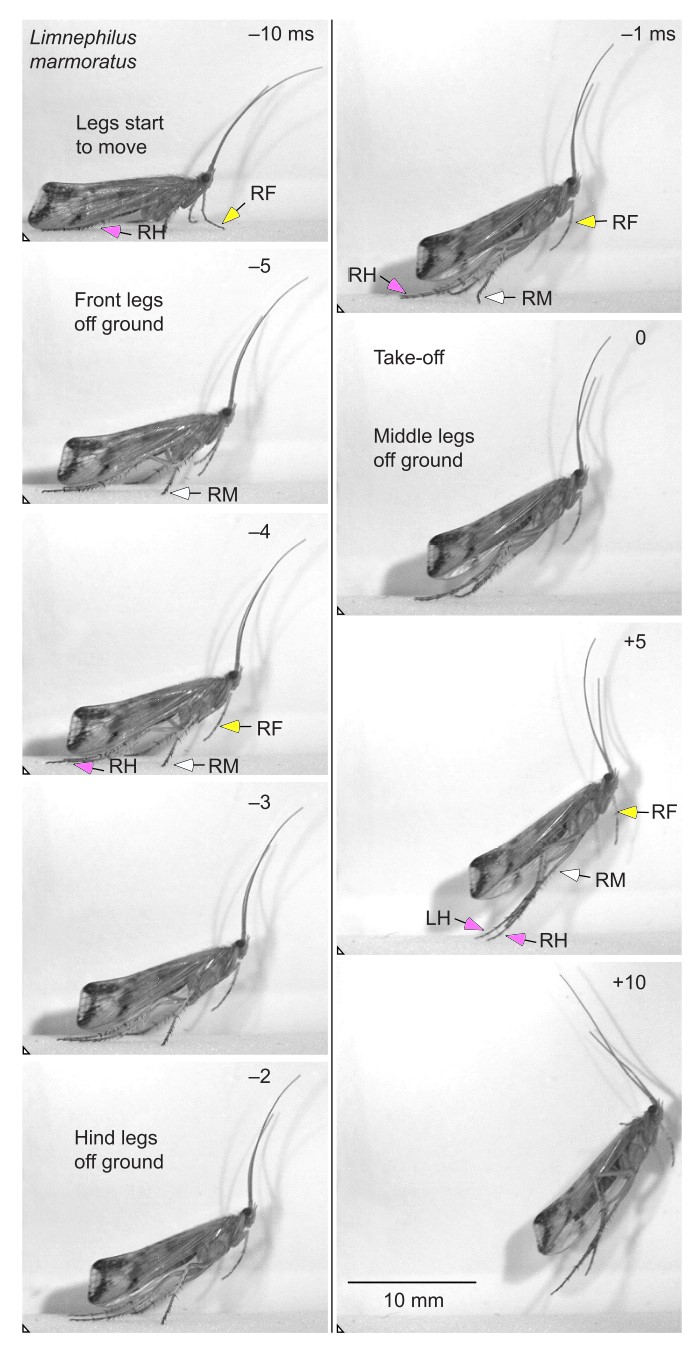
Kinematics
While two strategies for jumping are observed in caddis flies,they were measured to have a similar kinematic movement.
| Kinematic Metric | Caddis Flies performance |
|---|---|
| Duration | 17 ms |
| Max. Velocity | 1.1 m/s |
| Max. Acceleration | 64 m/s \(^2\) |
| Max. Power Output | 343 W/kg |
For further reading, Burrows and Dorosenko JEB 2015 describes the jumping mechanisms of caddis flies.
Snow Flea
Catapult jump mechanism
Snow fleas are flightless and live their adult lives during the winter in the northern hemisphere, moving by jumping and walking across snow.
Burrows (see: Burrows JEB 2011) examined the anatomy of the snow flea, as well as the kinematics of its jump, to gain a greater relationship between this species and fleas and proposed that snow fleas use a catapult mechanism.
Kinematics of the Jump
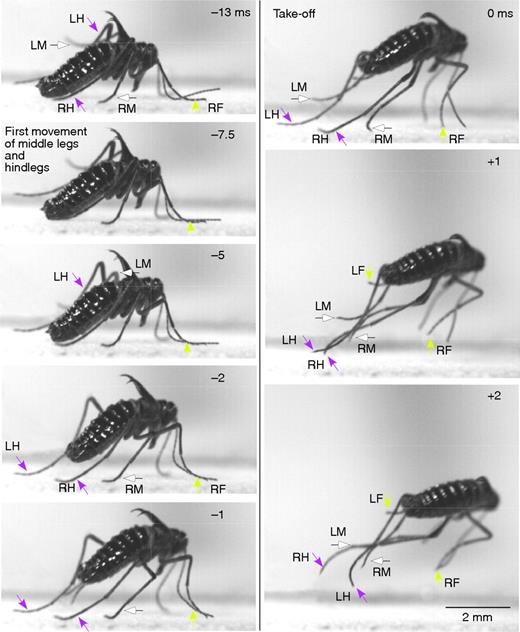
Fig. 3 from Burrows, showing the jump of a male snow flea, highlights the movements of the individual legs in the snow flea’s jump.
The jump starts from a standing, curved position. The middle and hind legs propel the jump, while the first seem not to be involved.The jump itself involves rotation of the back two sets of legs, with the first legs remaining stable
Energy Storage
The power per mass of muscles runs from 450 Wk kg^-1 for females to 740 W kg^-1, which is close to the upper limits of striated muscles, despite the sub-zero (Celsius) temperatures the snow fleas jump in. With temperature considerations, Burrows proposes that there must be an energy storage mechanism, which is corroborated by the presence of resilin, which is involved in energy storage with fleas.
For more information see: Burrows JEB 2011
Cone Snail
Ballistic Tooth Propulsion
Cone snails have extremely specialized teeth, which are a combination of torpedo and hypodermic needle. There are roughly 800 species of Cone Snail (Conidae), all carnivorous, which feed on a variety of fish, smaller cone snails, or other prey [1]. They hunt by shooting out a hollow, detachable tooth and injecting their prey with a neurotoxin, as shown in this PBS video. (Extensive research has also been done on the biochemistry of their neurotoxin “cocktails” [2].) They then usually swallow the prey whole.
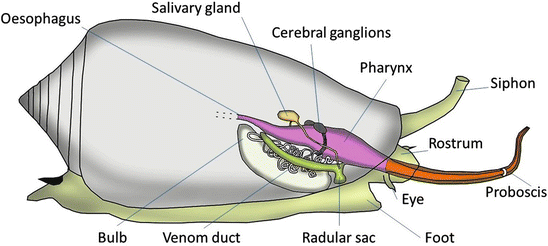

This Schulz et. al Current Biology 2019 paper describes the firing mechanism - the tooth is shaped like a harpoon, with a bulb at the proximal end. There is a muscular sphincter (round muscle) proximal to the tooth which likely pressurizes the lumen, and a constriction which acts as a latch for the tooth. This Salisbury et. al Journal of Experimental Biology 2010 paper found that the muscular sphincter may also control venom flow after the tooth punctures into the prey.
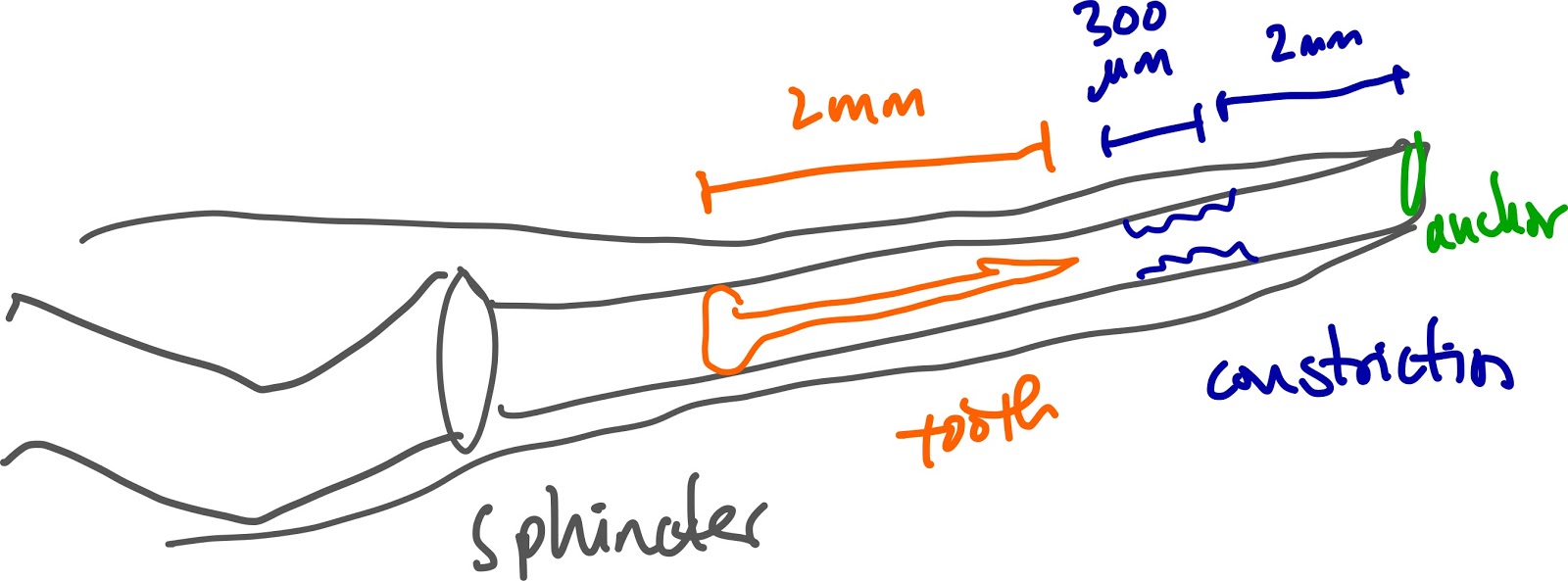
Fig. 4 from Salisbury et. al. Mechanism of prey capture in Conus pennaceus. Images of Conus pennaceus extending its proboscis towards the prey (a snail) in a recording channel. (A) Left column: sequential still frames taken from high-speed video (325 frames s–1) illustrating venom movement through the central lumen of the proboscis. Right column: diagrams illustrating venom movement through the proboscis lumen at various densities (light grayless dense, dark graymore dense) passing through the muscular sphincter. 0 ms indicates the frame immediately following radular tooth propulsion. Arrowheads indicate the location of the muscular sphincter. Asterisks mark the position of the base of the radular tooth. Arrows indicate the initial increase in lumenal kinking.
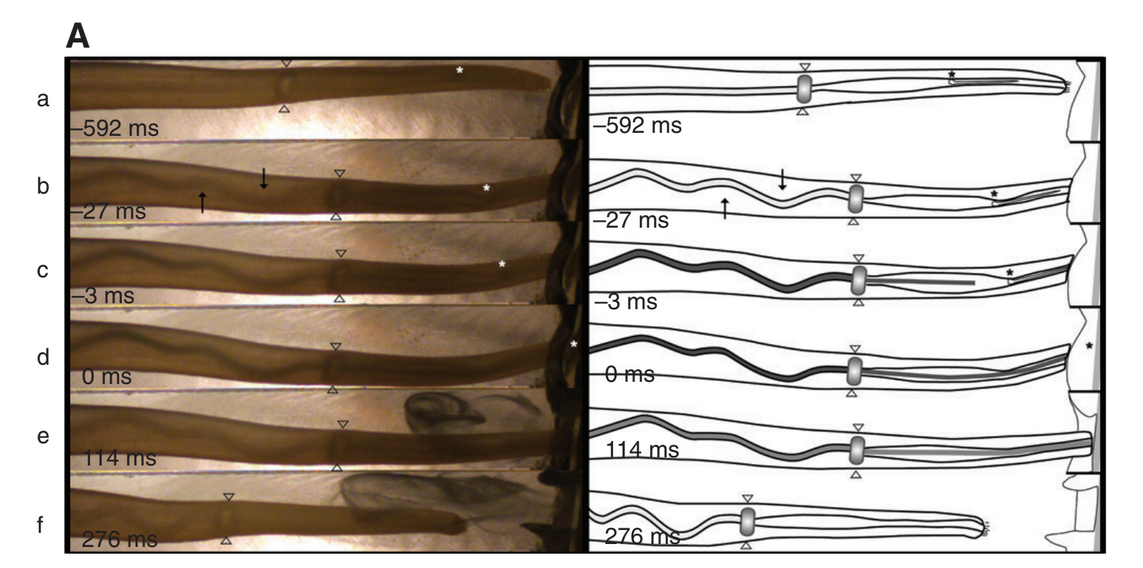
Fig. 1 from Schulz et. al. (C) Average tooth displacements over time during the prey strike (D) Average velocities during the prey strike (E) Average accelerations during the prey strike
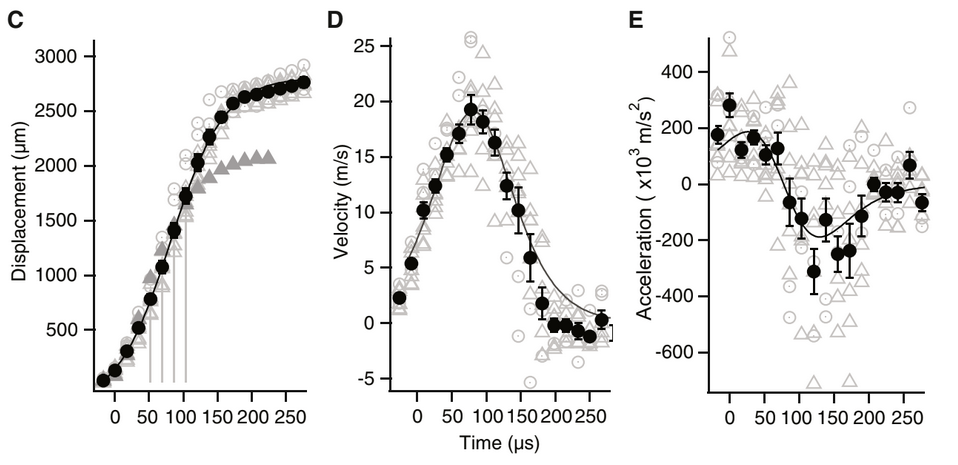
Summary of Kinematic Peformance from Schulz et al
| Kinematic Metric | Cone Snail performance |
|---|---|
| Duration | 100 μs |
| Max. Velocity | 25 m/s |
| Max. Acceleration | 400,000 m/s2 |
Sources Cited:
[3] Toto Olivera Cone Snail Neurotoxin Video
Snipefish
Pivot feeding
Lucas is editing Seahorses and pipefish have been shown to use elastic recoil to rotate their heads extremely quickly and use suction to capture prey. Snipefish, a species related to seahorses, also employ an elastic recoil system to capture prey on the scale of as quick as 2 milliseconds!
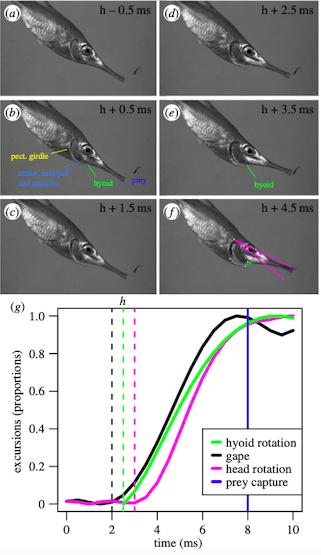
Figure 1: Screenshots from a snipefish feeding strike, along with the magnitude of movements before and during the strike.
Looking closer at the anatomy of snipefish, there are four distincts linkages that form a four-bar linkage system that acts as a latch that facilitates the quick release of energy. The four parts are the suspensorium-neurocranium link (pink in Figure 2), pectoral girdle link (yellow in Figure 2), urohyal bone and sternohyoideus muscle link (blue in Figure 2), and hyoid link (green in Figure 2).
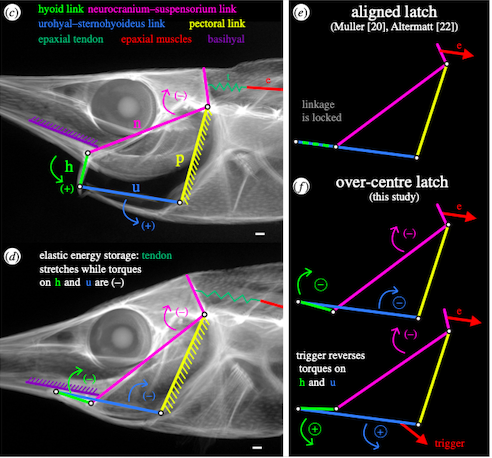
Figure 2: Links highlighted in the snipefish anatomy and converted into a four-bar linkage system
The idea of the hyoid link and urohyal-sternohyoideus link overlapping in the aligned latch as seen in the top right of Figure 2 was challenged by new studies that found that the latch system was an over-centre latch as seen in the bottom right of Figure 2, where the suspensorium-neurocranium link (pink) and urohyal bone and sternohyoideus muscle link (blue) overlap. The system becomes locked when the torque from the muscles pull the link towards the top of the head and stays there. Once the torque goes the other way, the system snaps quickly towards the other direction.
For more information see: Longo et al RSPB 2018
## Dracula Ant
Snap jaw
The Dracula Ant’s snap-jaw mandibles are used to hunt prey as well as for defensive purposes. Snap-jaws are a kind of power-amplified appendage as well as the fastest known animal appendage. Dracula Ants use their mandibles by sliding them together, which is similar to snapping fingers. The mandible that slides over the other mandible moves first, making the movement of the mandibles asynchronous. During this movement the mandible reaches peak velocity approximately halfway through its path. Unlike other accelerating appendages, the snap jaw includes the latch and spring in the accelerating mandible, instead of them being different parts. Dracula Ant’s are also known as the genus Mystrium, and in this genus the head and body size of species varies, which makes it convenient to test how the size of the mandibles affects the motion.
)
##### Fig. 1 Image of Dracula Ant (a) and Dracula Ant mandibles (b & c) from Larabee et RSOS 2018.
| Kinematic Metric | Dracula Ant Performance |
|---|---|
| Duration | 23 microseconds |
| Max. Velocity | 90 m/s |
Works Cited:
Nematode
Jump
Entomopathogenic nematodes are a soft bodied and legless animal that can jump a distance many times its own body length. They accomplish this by bending their body to store energy, and then releasing the stored energy very quickly. Jumping can also help nematodes ambush host insects, giving jumping nematodes an evolutionary advantage.1
Fig. 1 Mechanism of storing energy by creating and contracting a loop from Campbell and Kaya Can. J. Zool. 1999. (A) Steps of nematode jump. Frames 1-6 shows the nematode bending its body to form a loop. Frames 6-11 shows the nematode contracting the loop to store more energy. The label k indicates a kink in the cuticle of the nematode. (B) Illustration showing the timeframe for each step of jumping.
The nematode stores energy by contracting a loop made with its body (see in Fig 1). This loop is held together by surface tension of the film of water covering the nematode. Once the cuticle kinks, it has stored enough energy to jump and break the surface tension. Once the surface tension is broken, the stored energy is released in less than 30 milliseconds and the nematode is propelled forward.1 A summary of the kinematic performance is shown below:
| Kinematic Metric | Nematode performance |
|---|---|
| Duration | < 30 ms |
| Max. Velocity | 1.13 m/s |
| Max. Acceleration | 1609 m/s\(^2\) |